2. 南京林业大学生物与环境学院 南京 210037;
3. 南京晓庄学院 南京 211171
2. College of Biology and Environment, Nanjing Forestry University, Nanjing 210037, China;
3. Nanjing Xiaozhuang University, Nanjing 211171, China
水稻(Oryza sativa L.)是世界上重要的粮食作物之一。而干旱严重制约着水稻的生长和发育, 降低其产量[1]。近年来全球干旱呈加重趋势, 从多角度解析水稻的耐旱机理成为现代农业生物学研究的热点之一。C4植物如玉米(Zea mays L.)在干旱等逆境条件下表现较高的光合效率和水分利用率, 进而表现一定的抗逆性[2]。因此, 科学家们一直希望将C4植物中的关键基因转入C3植物水稻中, 提高水稻光合效率和耐逆性, 增加产量[3]。玉米磷酸烯醇式丙酮酸羧化酶(phosphoenolpyruvate carboxylase, PEPC, EC 4.1.1.31)是C4植物光合途径中的关键酶。PEPC不仅在提高C4光合效率中起关键作用, 在耐旱性方面也扮演着重要的角色[4]。现已通过基因工程技术获得高表达转玉米C4-pepc基因水稻(PC)[5-6]。众多研究表明PC水稻不仅表现出较强的光合能力和较高的产量[7-10], 而且表现耐旱以及耐低氮特性[11-15]。
PEPC参与植物光合作用中的碳固定以及三羧酸循环中的碳回补功能, 大量研究发现在各种生物胁迫和非生物胁迫下PEPC活性增加, 这有可能来源于基因的转录水平、蛋白质合成或蛋白质磷酸化水平的增加[16]。转玉米C4型pepc基因水稻在光氧化、高温和干旱胁迫下具有相对较高的光合速率, 叶绿素含量和成分衰减缓慢, 活性氧产生速率较低, PSⅡ活性较稳定, 表现出耐光氧化的特性[7-8, 17-20]。近年来的研究表明:干旱胁迫诱导PC引发多种信号转导机制, 如Ca2+(calcium ion)、H2O2(hydrogen peroxide)、NO(nitric oxide)和ATP(adenosine triphosphate)信号等[10, 21-24], 而且PC能够通过下游依赖性的蛋白激酶调控相关基因包括干旱诱导转录因子基因NAC6、bZIP60以及干旱诱导蛋白激酶基因CPK4、CPK9、SnRK1A、OsK24和OsK35等[19-24]。干旱胁迫能够快速诱导水稻干旱相关转录因子和蛋白激酶表达, 通过调控抗旱基因表达,磷酸化和去磷酸化调控机制, 响应干旱胁迫[25]。
光合产物的运输与分配对植物体的生长发育具有重要的作用, 是决定作物生长发育的重要生理过程。之前的研究证实在C3植物叶肉细胞中具有C4微循环的运转, 并且C3植物水稻中PEPC是C4微循环中的限速酶[26]。而二氯苯基二甲基脲[3-(3’, 4’-dich-lorophenyl)-1, 1-dimethyl-urea, DCMU]是一种除草剂(敌草隆), 也是一种电子传递抑制剂, 可以阻断类囊体膜上的电子传递, 从而抑制光合作用。它抑制从PSⅡ上的Q向PQ的电子传递, 从而可能抑制碳水化合物的累积。由于C4途径比C3途径要消耗更多的能量, DCMU可以结合在光反应中心D1蛋白的QB位点上, 阻止QB的还原, 从而阻断电子传递, 进而抑制光合作用[27-28]。
众所周知, 蔗糖不仅作为光合产物的主要形式, 而且在逆境条件下植物通过光合作用合成的碳更容易向次生代谢物转化, 其中花青素是重要的抗氧化分子, 有助于保护植物免受ROS(reactive oxygen species)的损害[29]。在许多植物物种中, 尤其是在逆境条件下, 花青素的积累受糖(sugar)和光信号传导正调节。如蔗糖(sucrose, Suc)是拟南芥[Arabidopsis thaliana (L.) Heynh.]幼苗中花青素生物合成最有效的诱导剂, 其他糖和渗透物质效果较差或无效[30]。在拟南芥中Suc特异性信号通路可进一步刺激果聚糖和花青素的产生, 而且Suc特异地诱导花青素生物合成需要MYB75/PAP1等转录因子基因的参与[31], MYB型转录因子在其生物合成中具有中心作用且其还严格依赖于Ca2+[29]; 小麦R2R3-MYB蛋白PURPLE PLANT1(TaPL1)作为花青素生物合成的正调节子, 参与花青素的合成[32]。已有研究表明SnRK激酶能够通过感知Ca2+、NO和ABA等信号激活, 从而通过MYB等转录因子调节花青素的合成[29, 31, 33-34]。但在水稻中糖是否诱导花青素生物合成以及参与信号的调节机制还不明确。但我们可以从高表达转玉米C4-pepc水稻前期研究中获得一些线索, 如本文之前的研究已经表明该材料耐旱, 且在PEG 6000模拟干旱处理下, PC水稻中Suc含量显著被上调; 此外, 本实验室之前在植株水平和细胞水平均证实其耐旱的响应过程依赖于细胞内的钙离子[22-23]。本研究选取干旱耐性以及光合能力差异明显的高表达转玉米C4-pepc基因水稻(PC)和未转基因的野生型水稻(WT)为材料, 在供试材料的4~5叶期, 采用50 μmol∙L-1 DCMU根吸入预处理1 h, 并采用12% PEG-6000对稻苗进行模拟干旱处理, 解析光合、糖和花青素参与PC水稻的干旱响应, 探究碳水化合物的积累和花青素合成之间的联系, 从而解析高光效和抗逆之间的协调关系, 期望更多地了解C4-pepc在水稻中缓解干旱胁迫的生理功能, 为“C4稻”在干旱中的应用提供理论依据。
1 材料与方法 1.1 供试材料试验以高表达转玉米C4-pepc基因水稻(PC)[5]和野生型水稻‘Kitaake’(WT)为试验材料。所选材料种子首先用75%的酒精洗涤浸泡5 min, 然后用50%次氯酸钠溶液浸种消毒15 min, 随后用蒸馏水反复冲洗数次直至无次氯酸钠残留。将种子置于30 ℃、黑暗条件下4 d, 然后使用国际水稻研究所(International Rice Research Institute)营养液配方进行培养[35]。待水稻长到5~6叶期, 于晴天傍晚选择株型、长势、叶片均一的植株进行处理, 并统一测定生理指标。
1.2 DCMU预处理和模拟干旱胁迫处理参照Qian等[22]的方法, 将所有植株分3组, 每组30株, 重复3次:一组加入等量含50 μmol∙L-1光合电子传递抑制剂DCMU溶液的营养液, 预处理1 h, 再加入含12% PEG-6000的营养液处理2 h; 一组加入营养液, 再加入含12% PEG-6000的营养液处理2 h; 另一组只加入营养液。在处理结束选取后, 随机选取5株的倒2叶, 快速进行样品采集, 用液氮速冻后, 立即置于-80 ℃储存备用。
1.3 光合参数的测定参照Li等[36]的方法, 采用美国LI-COR公司生产的LI-6400便携式光合测定仪, 利用仪器自带的红蓝光源, 大气CO2浓度400 μmol∙mol-1, 光量子通量密度(photosynthetic photon quanta flux density, PPFD)800 μmol∙m-2∙s-1, 流速设定为500 μmol∙s-1, 叶室温度设置为25 ℃。室内温度控制在25~30 ℃, 在各处理结束时取植株的倒2叶, 测定其净光合速率(net photosynthesis rate, Pn)、气孔导度(stomatal conductance, Gs)、胞间CO2浓度(intercellular CO2 concentration, Ci)及羧化效率(carboxylation efficiency, CE)等指标, 每个处理30株, 每次随机选择测量4次, 重复3~5次。
1.4 叶片相对含水量(relative water content, RWC)的测定使用打孔器在供试材料倒2叶的中间1/3处打取直径为0.5 cm的叶圆片10片, 称其鲜重(fresh weight, FW); 然后将叶圆片悬浮于装有蒸馏水的培养皿中, 4 ℃黑暗下处理18 h, 称饱和鲜重(turgid fresh weight, TW), 最后将叶圆片于105 ℃杀青15 min, 然后置于75 ℃下烘至恒重。最后用千分之一天平称其干重(dry weight, DW)。相对含水量(relative water content, RWC)按照如下公式计算: RWC(%)=(FW–DW)/(TW–DW)×100%。
1.5 可溶性糖及各糖组分含量的测定糖组分和含量测定参照Ambavaram等[37]的方法进行。可溶性糖、蔗糖、葡萄糖、果糖含量分别在620 nm、290 nm、505 nm和285 nm测定吸光值。
1.6 花青素含量的测定花青素含量的测定参照Rabino等[38]的方法进行, 分别在530 nm、657 nm处测定吸光值, 花青素含量的计算则通过A530-A657计算。
1.7 一氧化氮(NO)含量的测定水稻叶片NO浓度的测定参照Murphy等[39]的方法。
1.8 过氧化氢(H2O2)含量的测定水稻叶片H2O2浓度的测定参照Ren等[10]的方法。
1.9 钙离子(Ca2+)含量的测定水稻叶片Ca2+含量测定参照Yang等[40]方法。
1.10 磷酸烯醇式丙酮酸羧化酶(phosphoenolpyruv ate carboxylase, PEPC)活性的测定磷酸烯醇式丙酮酸羧化酶(PEPC, EC 4.1.1.31)酶活性测定参考Qian等[22]的方法。粗酶液提取, 取0.15 g叶片置于研钵冰浴研磨, 其中加入l.5 mL粗酶提取缓冲液[50 mmol∙L-1 Tris-HCl(三羟甲基)氨基甲烷(pH 7.8)], 5 mmol∙L-1二硫苏糖醇(Dithiothreitol, DTT), 1 mmol∙L-1氯化镁(MgCl2), 2%聚乙烯毗咯烷酮(m/v), 在4 ℃下12 000 r·min-1离心10 min, 收集上清液, 即为粗酶液。
酶活性测定反应体系(1 mL): 700 μL蒸馏水, 50 mmol∙L-1 Hepes-KOH (pH 8.0), 5 mmol∙L-1 MgCl2, 10 mmol∙L-1碳酸氢钠, 0.2 mmol∙L-1烟酰胺腺嘌呤二核苷酸(nicotinamide ade-nine dinucleotide, NAD), 1.5 U苹果酸脱氢酶(malate dehydrogenase), 150 μL粗酶液, 5 mmol∙L-1磷酸烯醇式丙酮酸(phosphoenolpy-ruvate, PEP), 将上述试剂摇匀后, 使用UV-1200紫外分光光度计, 在340 nm处记录吸光度的变化值, 计算酶活性。
1.11 总RNA的提取和实时荧光定量聚合酶链式反应[quantitative real-time (polymerase chain reaction, PCR), QRT-PCR]根据Jung等[41]的试验方法制备总RNA。反转录参考Chen等[42]的方法, 在PCR仪(ETC811, 北京东胜创新生物科技有限公司)上进行。根据制造商的说明书使用SYBR Premix Ex Taq Ⅱ试剂盒[TaKaRa Biotechnology(大连)有限公司], 进行QRT-PCR分析。使用Applied Biosystems Step One TM实时PCR系统(Applied Biosystems, Foster City, CA, USA)进行分析。QRT-PCR的反应条件依次为95 ℃ 10 min, 94 ℃ 30 s, 58 ℃ 40 s和68 ℃ 1 min, 共32个循环。每个试验重复3次。引物在Primer 3上设计, 以水稻组成性表达的ACTIN基因为内部参照, 引物序列如表 1所示。
| 表1 QRT-PCR的基因和引物 Table 1 Genes and primers for QRT-PCR |
使用SPSS18.0软件对数据进行One-Way ANOVA分析, 采用Microsoft Excel 2013对数据进行描述和作图。QRT-PCR数据使用2-ΔΔCt的方法进行分析。
2 结果与分析 2.1 DCMU处理对干旱胁迫下转C4-pepc水稻相对含水量及PEPC酶活性的影响图 1A显示, 在正常条件下, WT和PC水稻相对含水量没有显著差异, 使用12% PEG-6000模拟干旱胁迫处理2 h, 两种供试材料的相对含水量均显著下降, PC显著高于WT; 而DCMU联合12% PEG-6000处理进一步显著下调两种供试材料的相对含水量, 且PC仍显著高于WT (图 1A)。
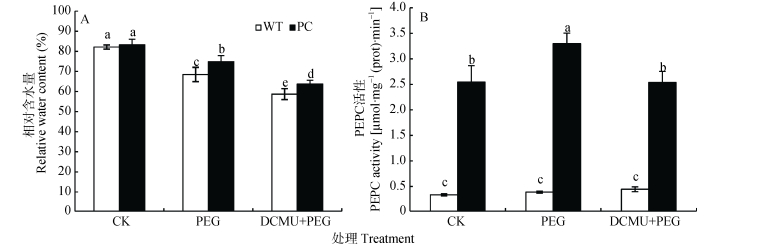
|
图 1 DCMU预处理对模拟干旱胁迫下高表达转C4-pepc基因水稻(PC)和野生型水稻(WT)叶片相对含水量(A)和PEPC活性(B)的影响 Figure 1 Effects of DCMU pretreatment on leaves relative water content (A) and PEPC activity (B) of C4-pepc gene overexpressed rice (PC) and untransformed wild-type rice (WT) under simulated drought stress CK:正常水培培养; PEG: PEG单独施加模拟干旱处理; DCMU+PEG: DCMU预处理之后联合模拟干旱胁迫处理。不同小写字母表示WT和PC的不同处理间差异显著(P<0.05)。 CK: normal hydroponic culture; PEG: PEG simulated drought stress; DCMU+PEG: DCMU pretreatment plus simulated drought stress. Different lowercase letters indicate significant differences among different treatments of WT and PC at P < 0.05. |
植物pepc基因在应对逆境胁迫中具有重要的作用, 高温、干旱以及盐胁迫都会导致PEPC酶活性及基因表达显著增加[8, 11, 43]。从图 1B来看, 12% PEG-6000处理下PC水稻中PEPC酶活性始终显著高于WT, 且DCMU联合12% PEG-6000处理会显著降低PC水稻中PEPC酶活性, 而WT水稻中PEPC活性在各处理中无显著变化(图 1B)。
2.2 DCMU处理对干旱胁迫下转C4-pepc水稻光合气体交换参数的影响如图 2A-D所示, 相比正常条件下, 12% PEG-6000模拟干旱胁迫均显著降低了PC与WT水稻的净光合速率、气孔导度、胞间CO2浓度以及羧化效率, 并且PC与WT之间存在显著差异(P<0.05)。同时, DCMU联合12% PEG-6000处理进一步下调PC与WT水稻的净光合速率、气孔导度和羧化效率, 且PC显著高于WT(P<0.05)(图 2A、B、D), 而胞间CO2浓度无显著性变化(图 2C), 说明DCMU根部吸入预处理使供试水稻的光合参数均显著下降, 且PC的始终高于WT, 提示除能量传递外, 可能还有其他因素参与了PC的耐旱机制。
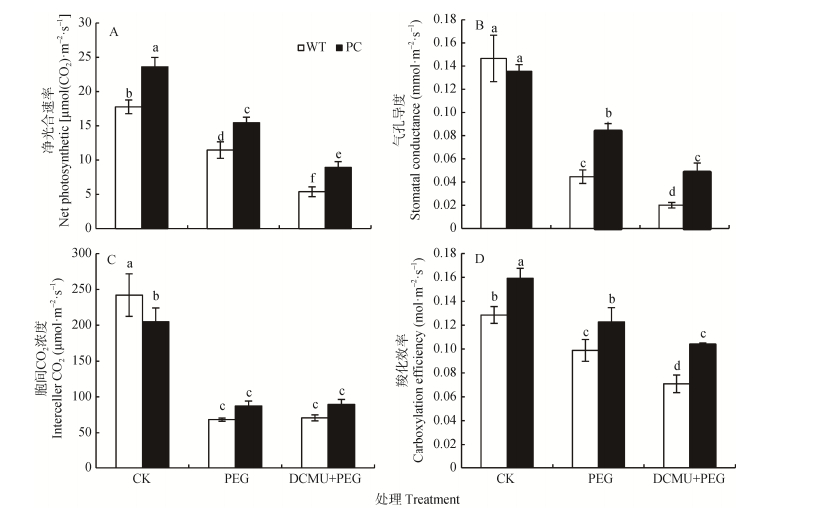
|
图 2 DCMU预处理对模拟干旱胁迫下高表达转C4-pepc基因水稻(PC)和野生型水稻(WT)叶片光合参数(A:净光合速率; B:气孔导度; C:胞间CO2浓度; D:羧化效率)的影响 Figure 2 Effects of DCMU pretreatment on photosynthetic parameters (A: net photosynthetic rate; B: stomatal conductance; C: intercellular CO2 concentration; D: carboxylation efficiency) of C4-pepc gene overexpressed rice (PC) and untransformed wild-type rice (WT) leaves under simulated drought stress CK:正常水培培养; PEG: PEG单独施加模拟干旱处理; DCMU+PEG: DCMU预处理之后联合模拟干旱胁迫处理。不同小写字母表示WT和PC的不同处理间差异显著(P<0.05, LSD test)。 CK: normal hydroponic culture; PEG: PEG simulated drought stress; DCMU+PEG: DCMU pretreatment plus simulated drought stress. Different lowercase letters indicate significant differences among different treatments of WT and PC at P < 0.05. |
在信号转导通路中, NO、H2O2和Ca2+都是植物中重要的信号分子[22-23]。由图 3A可知, NO含量在正常情况下PC显著高于WT, 使用12% PEG-6000模拟干旱胁迫处理2 h的水稻幼苗, PC与WT中NO含量均显著下降, 但PC仍显著高于WT; 而DCMU联合12% PEG-6000处理则显著上调两种供试材料的NO含量, 其中PC恢复到未处理的对照水平, 而DCUMU处理下WT含量仍显著低于WT未处理, 且PC显著高于WT。各处理条件下, PC水稻中H2O2含量始终低于WT(图 3B)。从图 3C可知, DCMU联合12% PEG-6000处理上调两种供试材料中Ca2+含量, 且PC显著高于WT。可见, 与单独PEG-6000模拟干旱胁迫相比, DCMU处理能够启动信号分子参与抗旱, 其中PC的变幅更为显著。
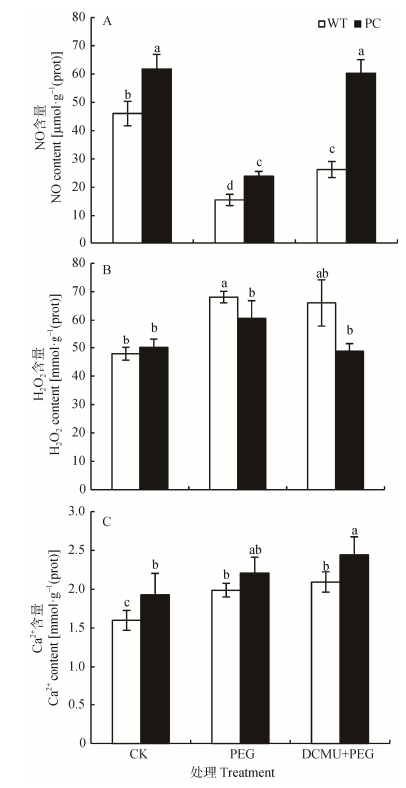
|
图 3 DCMU预处理对模拟干旱胁迫下高表达转C4-pepc基因水稻(PC)和野生型水稻(WT)叶片NO (A)、H2O2 (B)和Ca2+ (C)含量的影响 Figure 3 Effects of DCMU pretreatment on contents of NO (A), H2O2 (B) and Ca2+ (C) of C4-pepc gene overexpressed rice (PC) and untransformed wild-type rice (WT) leaves under simulated drought stress CK:正常水培培养; PEG: PEG单独施加模拟干旱处理; DCMU+PEG: DCMU预处理之后联合模拟干旱胁迫处理。不同小写字母表示WT和PC的不同处理间差异显著(P<0.05, LSD test)。 CK: normal hydroponic culture; PEG: PEG simulated drought stress; DCMU+PEG: DCMU pretreatment plus simulated drought stress. Different lowercase letters indicate significant differences among different treatments of WT and PC at P < 0.05. |
可溶性糖含量在干旱胁迫条件下的积累一方面有利于细胞渗透压的维持, 另一方面还可以作为糖信号调节其耐旱性[44]。干旱处理显著上调供试材料的可溶性糖含量, 但WT和PC之间无显著性差异(图 4A)。正常情况下PC水稻中蔗糖含量显著高于WT, 使用12% PEG-6000模拟干旱胁迫处理2 h的水稻幼苗, 均显著增加了WT和PC中蔗糖含量, 且PC显著高于WT; DCMU联合12% PEG-6000处理显著下调两种供试材料的蔗糖含量, 但仍未消除两个材料的差异, PC仍高于WT(图 4B)。模拟干旱处理也显著上调了供试材料中葡萄糖和果糖含量, 但DCMU处理对其没有影响, 且两供试材料之间没有显著差异(图 4C、D)。这些结果提示DCMU处理不仅影响了电子传递的能量, 还影响了物质代谢(可溶性糖的变化)。
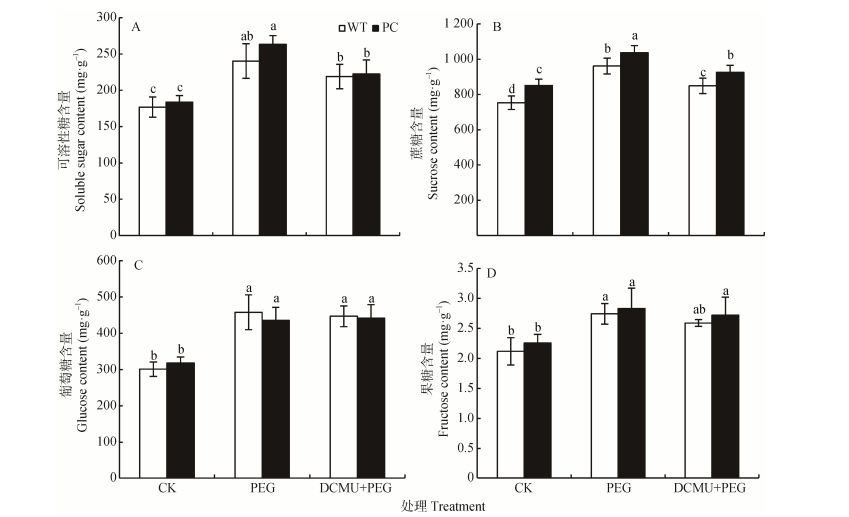
|
图 4 DCMU预处理对模拟干旱胁迫下高表达转C4-pepc基因水稻(PC)和野生型水稻(WT)叶片可溶性糖(A)、蔗糖(B)、葡萄糖(C)和果糖(D)含量的影响 Figure 4 Effects of DCMU pretreatment on contents of soluble sugar (A), sucrose (B), glucose (C) and fructose (D) of C4-pepc gene overexpressed rice (PC) and untransformed wild-type rice (WT) leaves under simulated drought stresses CK:正常水培培养; PEG: PEG单独施加模拟干旱处理; DCMU+PEG: DCMU预处理之后联合模拟干旱胁迫处理。不同小写字母表示WT和PC的不同处理间差异显著(P<0.05)。 CK: normal hydroponic culture; PEG: PEG simulated drought stress; DCMU+PEG: DCMU pretreatment plus simulated drought stress. Different lowercase letters indicate significant differences among different treatments of WT and PC at P < 0.05. |
虽然蔗糖对花青素合成的影响已有报道, 但在干旱条件下光合抑制剂处理后两者关系的研究少见报道。在试验过程中, 我们发现水稻花青素含量在使用12% PEG-6000模拟干旱胁迫处理之后, 其含量与对照相比显著上升, 且PC高于WT; 而DCMU和12% PEG-6000联合处理显著下调两种供试材料的花青素含量, 但PC仍显著高于WT(图 5)。干旱条件下, 花青素代谢是如何调控的?两种材料是否有差异?这是揭示两材料光合参数差异的关键。在水稻中, 花青素的生物合成途径主要是苯丙氨酸途径。其生物合成的相关基因主要有调节基因和结构基因2种。调节基因OsB1和OsB2与水稻叶片颜色有关, OsC1与水稻颖尖的颜色有关。而且, OsB1和OsB2编码bHLH转录因子, OsC1编码R2R3-MYB类转录因子, 它们和花青素合成相关基因一起共同调节花青素在水稻中的积累[45]。结构基因指直接编码花青素生物合成相关酶的基因, 主要有苯丙氨酸合成酶基因(phenylalnine ammonialyase, PAL)、查尔酮异构酶基因(chalcone isomerase, CHI)、查尔酮合成酶基因(chalcone synthase, CHS)、黄酮-3-氢化酶基因(flavonoid-3-hydroxylase, OsF3H)、黄酮-3’-氢化酶基因(flavonoid-3’-hydroxylase, F3’H)、二氢黄酮醇还原酶基因(dihydroflavonol-4-reductase, DFR)、花青素还原酶基因(anthocyanidin reductase, ANR)、花青素合成酶基因(anthocyanidin synthase, ANS)。我们进一步研究了12% PEG-6000处理和PEG-6000与DCMU联合处理分析花青素合成基因的变化情况。

|
图 5 DCMU预处理对模拟干旱胁迫下高表达转C4-pepc基因水稻(PC)和野生型水稻(WT)叶片花青素含量影响 Figure 5 Effect of DCMU pretreatment on anthocyanin content of C4-pepc gene overexpressed rice (PC) and untransformed wild-type rice (WT) leaves under simulated drought stress CK:正常水培培养; PEG: PEG单独施加模拟干旱处理; DCMU+PEG: DCMU预处理之后联合模拟干旱胁迫处理。不同小写字母表示WT和PC的不同处理间差异显著(P<0.05)。 CK: normal hydroponic culture; PEG: PEG simulated drought stress; DCMU+PEG: DCMU pretreatment plus simulated drought stress. Different lowercase letters indicate significant differences among different treatments of WT and PC at P < 0.05. |
图 6显示, OsB1、OsB2和OsC1在12% PEG-6000模拟干旱胁迫处理下表达量均显著上升, 且PC的表达量显著高于WT; 而DCMU与12% PEG-6000联合处理下两种供试材料的OsB1、OsB2和OsC1表达量下调, 但PC仍显著高于WT(图 5B-D)。
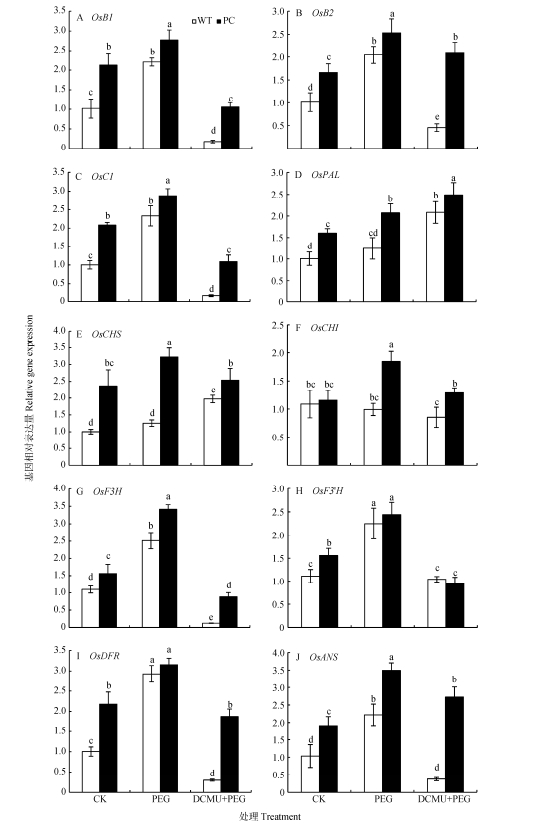
|
图 6 DCMU预处理对模拟干旱胁迫下高表达转C4-pepc基因水稻(PC)和野生型水稻(WT)叶片花青素合成酶基因表达的影响 Figure 6 Effects of DCMU pretreatment on expression of anthocyanin synthase genes of C4-pepc gene overexpressed rice (PC) and untransformed wild-type rice (WT) leaves under simulated drought stress CK:正常水培培养; PEG: PEG单独施加模拟干旱处理; DCMU+PEG: DCMU预处理之后联合模拟干旱胁迫处理。不同小写字母表示WT和PC的不同处理间差异显著(P<0.05, LSD test)。 CK: normal hydroponic culture; PEG: PEG simulated drought stress; DCMU+PEG: DCMU pretreatment plus simulated drought stress. Different lowercase letters indicate significant differences among different treatments of WT and PC at P < 0.05. |
如图 6所示, 正常条件下, PC中OsPAL、OsCHS、OsF3H、OsF3’H、OsDFR和OsANS表达量均高于WT(除OsCHI); 在12% PEG-6000模拟干旱胁迫处理下, PC中OsPAL、OsCHS、OsCHI、OsF3H、OsF3’H、OsDFR和OsANS表达量均显著上升; 而DCMU与12% PEG-6000联合处理后其表达量显著下降(除OsPAL), 但PC始终高于WT。总体而言, 在干旱条件下PC水稻中花青素合成相关基因更高的转录水平有利于合成更多的花青素。
2.6 DCMU处理对干旱胁迫下转玉米C4-pepc水稻花青素调节因子的影响供试材料在模拟干旱条件下, 在转录水平上引起花青素调节和合成相关基因的表达差异, 结合本研究观察到第二信使分子NO含量分析的变化, 提示可能存在涉及花青素靶基因上游转录因子的变化。已经有研究表明:除了MBW(MYB-BHLH-WD40)转录因子以外, 已知COP1(constitutively photomorphogenic 1)和HY5(elongated hypocotyl 5)也是花青素调节中的两个重要的转录因子[46-47], 为此, 在本研究中进一步分析了其基因的表达。由图 7A可知, 在正常情况下, PC中OsCOP1显著高于WT; 12% PEG-6000模拟干旱胁迫处理下, 两种供试材料的OsCOP1均显著升高, 且PC显著高于WT; 而DCMU和12% PEG-6000联合处理下调了两种供试材料的OsCOP1表达量, 但PC仍显著高于WT。在正常条件下, 两种供试材料的OsHY5表达量无显著差异, 在12% PEG-6000模拟干旱胁迫处理下, PC中OsHY5显著上升, 且PC显著高于WT; 而DCMU和12% PEG-6000联合处理下调PC的OsHY5表达量, 但PC仍显著高于WT(图 6B)。综上, 在干旱条件下, PC可能通过第二信号分子NO和Ca2+的信号传导过程, 诱导了转录因子的变化, 从而增强花青素的积累。
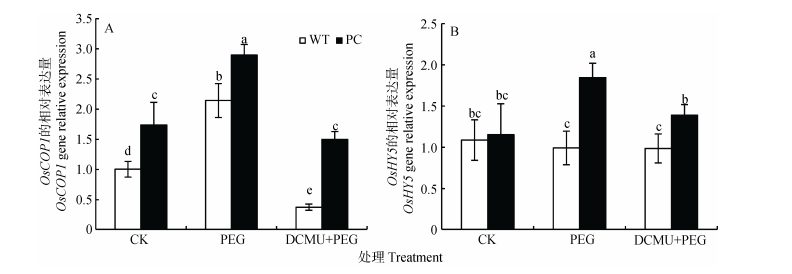
|
图 7 DCMU预处理对模拟干旱胁迫下高表达转C4-pepc基因水稻(PC)和野生型水稻(WT)叶片花青素调节因子的影响 Figure 7 Effect of DCMU pretreatment on regulators of anthocyanin of C4-pepc gene overexpressed rice (PC) and untransformed wild-type rice (WT) leaves under simulated drought stress CK:正常水培培养; PEG: PEG单独施加模拟干旱处理; DCMU+PEG: DCMU预处理之后联合模拟干旱胁迫处理。不同小写字母表示WT和PC的不同处理间差异显著(P<0.05, LSD test)。 CK: normal hydroponic culture; PEG: PEG simulated drought stress; DCMU+PEG: DCMU pretreatment plus simulated drought stress. Different lowercase letters indicate significant differences among different treatments of WT and PC at P < 0.05. |
在水稻生长过程中会遭受到各种环境胁迫, 例如高温、高光、干旱、盐和生物胁迫等影响。干旱胁迫引起气孔关闭, 胞间CO2浓度降低, 碳同化速率降低, 叶片吸收的光能过剩, 造成光合器官的光化学效率及光合速率降低, 发生光抑制甚至光破坏, 导致作物光合能力降低, 最终导致作物减产[48]。光合机构对干旱造成的水分胁迫有一定的耐受性, 通过光呼吸和热耗散增加等许多途径耗散掉多余的激发能, 以有效缓解光合作用中光抑制所产生的不良影响, 从而形成多种保护机制[49]。
在气孔开放期间, 高表达转玉米C4型pepc基因水稻的保卫细胞中PEPC可特异性调控导致苹果酸或草酰乙酸积累, 促进气孔开放, 增强光合作用[50]。3, 3-二氯-二羟基膦酰基-甲基-2-丙烯酸酯(DCDP, 3, 3-dichloro-2-dihydroxyphosphinoyl-methyl-2-propenoate) (PEPC的抑制剂)存在条件下, 可限制气孔开放[51-52]。而使用光合作用的抑制剂DCMU能够阻断PSⅡ中QA和QB之间的电子流动, 改变植株的能量状态而影响光合能力。已有研究表明, DCMU对植株光合过程的影响是通过不同的光质造成的, 通过气孔对不同波长光的反应发现DCMU在红光下可以抑制光合作用的进行, 而在蓝光下却促进了光合作用[53]。在红光下, 促进保卫细胞的叶绿体向细胞质中提供ATP, 这些ATP是质膜上的H+-ATPase形成质子泵和气孔张开所必需的[54], 可见, DCMU是通过调节植物体内的能量状态而影响光合的。前期的研究表明, 在干旱胁迫条件下, 与原种相比, PEPC的高表达能够维持较高的PSⅡ活性, 也产生较高的ATP, 来维持净光合速率的稳定[13]。本试验通过在模拟干旱胁迫条件下通过外源添加DCMU到高表达转C4-pepc基因水稻植株, 结果显示: PC中净光合速率、气孔导度、胞间CO2及羧化效率在DCMU处理之后仍显著高于WT, 且DCMU处理使得PC水稻中PEPC酶活性显著下降, 但PC中PEPC酶活性始终高于WT, 说明两供试材料能量状态的差异是导致其干旱表现的重要原因, 但还存在其他的机制。本研究发现供试材料中总可溶性糖和内源蔗糖含量变化趋势与光合参数类似。已有研究表明:在逆境条件下, 蔗糖可参与诱导花青素的积累[55]。在多种植物中, 花青素积累的组织特异性已被证明通过R2R3-MYB、bHLH和WD40转录因子3大类基因家族调节[29]。由MYB、bHLH和WD40所形成的三元复合体(MBW复合体), 其功能已经在模式植物拟南芥中得到详细阐述。本研究涉及MYB和bHLH两个转录因子, 其中OsC1编码R2R3-MYB类转录因子, OsB1和OsB2编码bHLH转录因子, 有文献表明OsB1和OsB2必需与MYB类转录因子互作才能发挥功能[56]。从结果来看PC水稻中OsC1、OsB1和OsB2在逆境条件下均显著高于WT, 而DCMU联合12% PEG-6000处理之后其转录水平显著下降, 但PC始终显著高于WT。而对于WD40, 已经在拟南芥、玉米、苜蓿(Medicago sativa L.)、甘薯[Dioscorea esculenta (Lour.) Burkill L.]等中报道过WD40类花色苷转录因子[30, 57], 而WD40在水稻响应非生物逆境的研究有限。本研究表明:花青素含量、花青素合成基因以及调节它的转录因子的表达也与蔗糖的变化相一致, 如OsB1、OsB2、OsC1、OsPAL、OsCHI、OsCHS、OsF3H、OsF3’H、OsDFR、OsANS、OsCOP1、OsHY5转录水平在模拟干旱条件下呈现出同步变化趋势, 提示蔗糖可能参与花青素的调节和合成基因的表达。有研究表明蔗糖与植物抗逆的调节与Ca2+、NO以及H2O2等信号有非常密切的联系[34, 58], 本研究的确也观察到两材料在不同处理下, 其体内第二信使分子含量如Ca2+、NO以及H2O2的差异, 其中DCMU处理对NO含量的影响最为显著, 而且加剧了供试材料的差异表现, 说明PC可能通过NO参与蔗糖依赖的花青素积累的调节, 有关信息还需要今后NO与花青素的直接证据验证。
已知植物花青素的合成是一个需光过程, 在光条件下, 光受体接收光信号, 通过信号转导形成细胞内第二信使系统级联传递来调节包括花青素合成在内的光形态建成反应[59]。有研究表明: E3泛素连接酶组成型光形态建成(COP1)是位于光受体下游一个光形态建成的抑制子, 是一个光调控植物生长发育的分子开关。在暗条件下, COP1抑制光反应。COP1/SPA复合体与MYB调控因子互作调控花青素合成的结构基因的表达, 从而影响花青素的合成[59], 此外, 黑暗条件下植物光形态建成调控因子COP1积累在细胞核内, 直接与碱性亮氨酸拉链(bZIP)类转录因子HY5相互作用, 并被蛋白酶降解, 负调控下游基因的表达; 而在光下COP1从细胞核内转移到细胞核外, 从而HY5在细胞核内积累, 可特异结合于查尔酮合成酶基因CHS等光诱导基因启动子上, 正调控相关基因的表达[60]。进而, 本文对转录因子OsHY5也进行了研究, 从本研究结果可以看到, 在12% PEG-6000模拟干旱条件下, 在PC中OsCOP1和OsHY5的转录表达与MYB和bHLH转录因子呈现出一致的结果, 在逆境条件下均显著高于WT, 而DCMU联合12% PEG-6000处理之后其转录水平显著下降, 但PC始终显著高于WT。同时, 编码植物花青素的生物合成的7个基因中除OsPAL以外OsCHI、OsCHS、OsF3H、OsF3’H、OsDFR、OsANS与转录因子呈现出相一致的表达水平。
综上所述, 在干旱条件下, PC材料一方面可通过电子传递的能量差异应对干旱逆境, 还可以通过内源蔗糖含量的差异, 并通过第二信使Ca2+、NO感受干旱信号, 参与转录因子的调节(比如OsCOP1和OsHY5), 进而参与花青素合成基因的调控, 合成较多的花青素, 从而增强PC水稻对干旱逆境的响应。总之, 本研究提供了水稻中花青苷合成调控的新线索, 但蔗糖如何诱导PC的花青素调节相关的转录因子响应干旱的精确机制, 还需要今后的直接证据证实。
| [1] | CHAVES M M, FLEXAS J, PINHEIRO C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell[J]. Annals of Botany, 2009, 103(4): 551–560. DOI:10.1093/aob/mcn125 |
| [2] | ZHU X G, LONG S P, ORT D R. Improving photosynthetic efficiency for greater yield[J]. Annual Review of Plant Biology, 2010, 61: 235–261. DOI:10.1146/annurev-arplant-042809-112206 |
| [3] | KARKI S, RIZAL G, QUICK W P. Improvement of photosynthesis in rice (Oryza sativa L.) by inserting the C4 pathway[J]. Rice, 2013, 6(1): 28. DOI:10.1186/1939-8433-6-28 |
| [4] | O'LEARY B, PARK J, PLAXTON W C. The remarkable diversity of plant PEPC (phosphoenolpyruvate carboxylase): recent insights into the physiological functions and post-translational controls of non-photosynthetic PEPCs[J]. Biochemical Journal, 2011, 436(1): 15–34. DOI:10.1042/BJ20110078 |
| [5] | KU M S B, AGARIE S, NOMURA M, et al. High-level expression of maize phosphoenolpyruvate carboxylase in transgenic rice plants[J]. Nature Biotechnology, 1999, 17: 76–80. DOI:10.1038/5256 |
| [6] | JEANNEAU M, GERENTES D, FOUEILLASSAR X, et al. Improvement of drought tolerance in maize: towards the functional validation of the Zm-Asr1 gene and increase of water use efficiency by over-expressing C4-PEPC[J]. Biochimie, 2002, 84: 1127–1135. DOI:10.1016/S0300-9084(02)00024-X |
| [7] | JIAO D M, KUANG T Y, LI X, et al. Physiological characteristics of the primitive CO2 concentrating mechanism in PEPC transgenic rice[J]. Science in China Series C: Life Sciences, 2003, 46(4): 438–446. DOI:10.1360/02yc0075 |
| [8] | BANDYOPADHYAY A, DATTA K, ZHANG J, et al. Enhanced photosynthesis rate in genetically engineered indica rice expressing PEPC gene cloned from maize[J]. Plant Science, 2007, 172(6): 1204–1209. DOI:10.1016/j.plantsci.2007.02.016 |
| [9] | LIAN L, WANG X W, ZHU Y S, et al. Physiological and photosynthetic characteristics of indica Hang2 expressing the sugarcane PEPC gene[J]. Molecular Biology Reports, 2014, 41: 2189–2197. DOI:10.1007/s11033-014-3070-4 |
| [10] | REN C G, LI X, LIU X L, et al. Hydrogen peroxide regulated photosynthesis in C4-pepc transgenic rice[J]. Plant Physiology and Biochemistry, 2014, 74: 218–229. DOI:10.1016/j.plaphy.2013.11.011 |
| [11] | DING Z S, SUN X F, HUANG S H, et al. Response of photosynthesis to short-term drought stress in rice seedlings overexpressing C4 phosphoenolpyruvate carboxylase from maize and millet[J]. Photosynthetica, 2015, 53(4): 481–488. DOI:10.1007/s11099-015-0126-1 |
| [12] |
钱宝云, 刘小龙, 李霞. 钙肥对不同内源钙含量水稻品种光合参数的影响[J]. 江苏农业学报, 2014, 30(3): 467–473.
QIAN B Y, LIU X L, LI X. Photosynthesis of rice cultivars with various endogenous calcium contents in response to calcium fertilizer application[J]. Jiangsu Journal of Agricultural Sciences, 2014, 30(3): 467–473. |
| [13] |
霍垲, 陆巍, 李霞. 干旱胁迫下调节ATP的含量对提高转玉米C4型pepc水稻光合速率的影响[J]. 中国生态农业学报, 2015, 23(5): 605–613.
HUO K, LU W, LI X. Effect of regulating ATP on improving photosynthetic rate of transgenic rice with overexpressing maize C4 pepc under drought stress[J]. Chinese Journal of Eco-Agriculture, 2015, 23(5): 605–613. |
| [14] |
唐玉婷, 李霞, 陆巍, 等. 高表达转C4型PEPC基因水稻在低氮下诱导碳氮酶稳定光合作用[J]. 华北农学报, 2015, 30(4): 95–100.
TANG Y T, LI X, LU W, et al. Transgenic rice with high expression of C4-PEPC genes induced higher carbon and nitrogen key enzyme to maintain photosynthesis under low nitrogen conditions[J]. Acta Agriculturae Boreali-Sinica, 2015, 30(4): 95–100. DOI:10.7668/hbnxb.2015.04.017 |
| [15] | JIAO D M, XIA L, HUANG X Q, et al. The characteristics of CO2 assimilation of photosynthesis and chlorophyll fluorescence in transgenic PEPC rice[J]. Science Bulletin, 2001, 46(13): 1080–1084. DOI:10.1007/BF02900682 |
| [16] | DOUBNEROVá V, RYšLAVá H. What can enzymes of C4, photosynthesis do for C3, plants under stress?[J]. Plant Science, 2011, 180(4): 575–583. DOI:10.1016/j.plantsci.2010.12.005 |
| [17] | JIAO D M, LI X, JI B H. Photoprotective effects of high level expression of C4, phosphoenolpyruvate carboxylase in transgenic rice during photoinhibition[J]. Photosynthetica, 2005, 43(4): 501–508. DOI:10.1007/s11099-005-0082-2 |
| [18] |
李霞, 任承钢. ABA、BA及DPI对高表达玉米C4 pepc基因的水稻光合特性及叶绿素荧光特性的影响[J]. 植物生理学报, 2012, 48(6): 549–556.
LI X, REN C G. Effects on photosynthetic and fluorescence characteristics under treatments of ABA, BA or DPI in transgenic rice with over-expression C4-pepc gene[J]. Plant Physiology Journal, 2012, 48(6): 549–556. |
| [19] | LIU X, LI X, ZHANG C, et al. Phosphoenolpyruvate carboxylase regulation in C4-PEPC-expressing transgenic rice during early responses to drought stress[J]. Physiologia Plantarum, 2017, 159(2): 178–200. DOI:10.1111/ppl.2017.159.issue-2 |
| [20] | HUO K, LI X, HE Y F, et al. Exogenous ATP enhance signal response of suspension cells of transgenic rice (Oryza sativa L.) expressing maize C4-pepc encoded phosphoenolpyruvate carboxylase under PEG treatment[J]. Plant Growth Regulation, 2016, 82(1): 55–67. |
| [21] | QIAN B Y, LI X, LIU X L, et al. Enhanced drought tolerance in transgenic rice over-expressing of maize C4 phosphoenolpyruvate carboxylase gene via NO and Ca2+[J]. Journal of Plant Physiology, 2015, 175: 9–20. DOI:10.1016/j.jplph.2014.09.019 |
| [22] | QIAN B Y, LI X, LIU X L, et al. Improved oxidative tolerance in suspension-cultured cells of C4-pepc transgenic rice by H2O2 and Ca2+ under PEG-6000[J]. Journal of Integrative Plant Biology, 2015, 57(6): 534–549. DOI:10.1111/jipb.12283 |
| [23] | NAKASHIMA K, TRAN L S P, DONG V N, et al. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice[J]. Plant Journal, 2007, 51: 617–630. DOI:10.1111/j.1365-313X.2007.03168.x |
| [24] | NIJHAWAN A, JAIN M, TYAGI A K, et al. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice[J]. Plant Physiology, 2008, 146(2): 333–350. |
| [25] | HUANG G T, MA S L, BAI L P, et al. Signal transduction during cold, salt, and drought stresses in plants[J]. Molecular Biology Reports, 2012, 39: 969–987. DOI:10.1007/s11033-011-0823-1 |
| [26] |
朱素琴, 季本华, 焦德茂. 亚硫酸氢钠对转PEPC基因水稻叶片光合作用的促进作用[J]. 科技通报, 2004, 20(6): 523–528.
ZHU S Q, JI B H, JIAO D M. Promotive effect of NaHSO3 on photosynthesis in PEPC transgenic rice leaves[J]. Bulletin of Science and Technology, 2004, 20(6): 523–528. |
| [27] | BISWAL A K, DILNAWAZ F, DAVID K A V, et al. Increase in the intensity of thermoluminescence Q-band during leaf ageing is due to a block in the electron transfer from QA to QB[J]. Luminescence, 2001, 16(5): 309–313. DOI:10.1002/(ISSN)1522-7243 |
| [28] | SUZUKI K, OHMORI Y, RATEL E. High root temperature blocks both linear and cyclic electron transport in the dark during chilling of the leaves of rice seedlings[J]. Plant and Cell Physiology, 2011, 52(9): 1697–1707. DOI:10.1093/pcp/pcr104 |
| [29] | VAN DEN ENDE W, EL-ESAWE S K. Sucrose signaling pathways leading to fructan and anthocyanin accumulation: A dual function in abiotic and biotic stress responses?[J]. Environmental and Experimental Botany, 2014, 108: 4–13. DOI:10.1016/j.envexpbot.2013.09.017 |
| [30] | TENG S, KEURENTJES J, BENTSINK L, et al. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene[J]. Plant Physiology, 2005, 139(4): 1840–1852. DOI:10.1104/pp.105.066688 |
| [31] | SANYAL S K, PANDEY A, PANDEY G K. The CBL-CIPK signaling module in plants: a mechanistic perspective[J]. Physiologia Plantarum, 2015, 155: 89–108. DOI:10.1111/ppl.2015.155.issue-2 |
| [32] | SHIN D H, CHOI M G, KANG C S, et al. A wheat R2R3-MYB protein PURPLE PLANT1 (TaPL1) functions as a positive regulator of anthocyanin biosynthesis[J]. Biochemical & Biophysical Research Communications, 2016, 469(3): 686–691. |
| [33] | YASUDA S, AOYAMA S, HASEGAWA Y, et al. Arabidopsis CBL-interacting protein kinases regulate carbon/nitrogen-nutrient response by phosphorylating ubiquitin ligase ATL31[J]. Molecular Plant, 2017, 4(10): 537–658. |
| [34] | SANYAL S K, KANWAR P, YADAV A K, et al. Arabidopsis CBL interacting protein kinase 3 interacts with ABR1, an APETALA2 domain transcription factor, to regulate ABA responses[J]. Plant Science, 2017, 254: 48–59. DOI:10.1016/j.plantsci.2016.11.004 |
| [35] | YOSHIDA S, FORNO D A, COCK J H, et al. Laboratory Manual for Physiological Studies of Rice[M]. Philippines: International Rice Research Institute, 1976: 61-64. |
| [36] | LI X, WANG C, REN C G. Effects of 1-butanol, neomycin and calcium on the photosynthetic characteristics of PEPC transgenic rice[J]. African Journal of Biotechnology, 2011, 10: 17466–17476. |
| [37] | AMBAVARAM M M R, BASU S, KRISHNAN A, et al. Coordinated regulation of photosynthesis in rice increases yield and tolerance to environmental stress[J]. Nature Communications, 2014, 5: 1–14. |
| [38] | RABINO I, MANCINELLI A L. Light, temperature, and anthocyanin production[J]. Plant Physiology, 1986, 81(3): 922–924. DOI:10.1104/pp.81.3.922 |
| [39] | MURPHY M E, NOACK E. Nitric oxide assay using hemoglobin method[J]. Methods in Enzymology, 1994, 233: 240–250. DOI:10.1016/S0076-6879(94)33027-1 |
| [40] | YANG C Q, LIU W N, ZHAO Z H, et al. Determination of the content of serum calcium with methylthymol blue as chromogenic reagent[J]. Spectroscopy & Spectral Analysis, 1998, 18: 485–487. |
| [41] | JUNG H, KIM J K, HA S W. Use of animal viral IRES sequence makes multiple truncated transcripts without mediating polycistronic expression in rice[J]. Journal of the Korean Society for Applied Biological Chemistry, 2011, 54: 678–684. DOI:10.1007/BF03253145 |
| [42] | CHEN P B, LI X, HUO K, et al. Promotion of photosynthesis in transgenic rice over-expressing of maize C4 phosphoenolpyruvate carboxylase gene by nitric oxide donors[J]. Journal of Plant Physiology, 2014, 171: 458–466. DOI:10.1016/j.jplph.2013.11.006 |
| [43] | JIA X H, ZHANG P P, SHI D J, et al. Regulation of PEPC gene expression in Anabaena sp. PCC 7120 and its effects on cyclic electron flow around photosystem I and tolerances to environmental stresses[J]. Journal of Integrative Plant Biology, 2015, 57(5): 468–476. DOI:10.1111/jipb.v57.5 |
| [44] | KEUNEN E, PESHEV D, VANGRONSVELD J, et al. Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept[J]. Plant Cell & Environment, 2013, 36(7): 1242–1255. |
| [45] | LORETI E, POVERO G, NOVI G, et al. Gibberellins, jasmonate and abscisic acid modulate the sucrose-induced expression of anthocyanin biosynthetic genes in Arabidopsis[J]. New Phytologist, 2008, 179(4): 1004–1016. DOI:10.1111/nph.2008.179.issue-4 |
| [46] | ZHANG Y C, GONG S F, LI Q H, et al. Functional and signaling mechanism analysis of rice CRYPTOCHROME 1[J]. Plant Journal, 2006, 46(6): 971–983. DOI:10.1111/tpj.2006.46.issue-6 |
| [47] | SHIN D H, CHOI M, KIM K, et al. HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis[J]. FEBS letters, 2013, 587(10): 1543–1547. DOI:10.1016/j.febslet.2013.03.037 |
| [48] |
丁在松, 周宝元, 孙雪芳, 等. 干旱胁迫下PEPC过表达增强水稻的耐强光能力[J]. 作物学报, 2012, 38(2): 285–292.
DING Z S, ZHOU B Y, SUN X F, et al. High light tolerance is enhanced by overexpressed PEPC in rice under drought stress[J]. Acta Agronomica Sinica, 2012, 38(2): 285–292. |
| [49] | SANTOS M G, RIBEIRO R V, MACHADO E C, et al. Photosynthetic parameters and leaf water potential of five common bean genotypes under mild water deficit[J]. Biologia Plantarum, 2009, 53(2): 229–236. DOI:10.1007/s10535-009-0044-9 |
| [50] | VAVASSEUR A, RAGHAVENDRA A S. Guard cell metabolism and CO2 sensing[J]. New Phytologist, 2005, 16: 665–682. |
| [51] | ZHANG C, LI X, HE Y F, et al. Physiological investigation of C4-phosphoenolpyruvate-carboxylase-introduced rice line shows that sucrose metabolism is involved in the improved drought tolerance[J]. Plant Physiology and Biochemistry, 2017, 115: 328–342. DOI:10.1016/j.plaphy.2017.03.019 |
| [52] | JENKINS C L D. Effects of the phosphoenolpyruvate carboxylase inhibitor 3, 3-dichloro-2-(dihydroxy phosphinoyl methyl) propenoate on photosynthesis. C4 selectivity and studies on C4 photosynthesis[J]. Plant Physiology, 1989, 89(4): 1231–1237. DOI:10.1104/pp.89.4.1231 |
| [53] | AGBARIAH K T, ROTHBEJERANO N. The effect of blue light on energy levels in epidermal strips[J]. Physiologia Plantarum, 1990, 78(1): 100–104. DOI:10.1111/ppl.1990.78.issue-1 |
| [54] | TOMINAGA M, KINOSHITA T, SHIMAZAKI K. Guard-cell chloroplasts provide ATP required for H+ pumping in the plasma membrane and stomatal opening[J]. Plant & Cell Physiology, 2001, 42(8): 795–802. |
| [55] | MENG L S, LI Y Q, LIU M Q, et al. The arabidopsis ANGUSTIFOLIA3-YODA gene cascade induces anthocyanin accumulation by regulating sucrose levels[J]. Frontiers in Plant Science, 2016, 7: 1728. |
| [56] | SAKAMOTO W, OHMORI T, KAGEYAMA K, et al. The purple leaf (Pl) locus of rice: The Pl(w) allele has a complex organization and includes two genes encoding basic helix-loop-helix proteins involved in anthocyanin biosynthesis[J]. Plant & Cell Physiology, 2001, 42(9): 982–991. |
| [57] | DONG W, NIU L L, GU J T, et al. Isolation of a WD40-repeat gene regulating anthocyanin biosynthesis in storage roots of purple-fleshed sweet potato[J]. Acta Physiologiae Plantarum, 2014, 36(5): 1123–1132. DOI:10.1007/s11738-014-1487-y |
| [58] | TOSSI V, AMENTA M, LAMATTINA L, et al. Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway[J]. Plant, Cell & Environment, 2011, 34(6): 909–921. |
| [59] | YIN R H, SKVORTSOVA M Y, LOUBéRY S, et al. COP1 is required for UV-B-induced nuclear accumulation of the UVR8 photoreceptor[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(30): E4415–E4422. DOI:10.1073/pnas.1607074113 |
| [60] | SRIVASTAVA A K, SENAPATI D, SRIVASTAVA A, et al. Short hypocotyl in white light1 interacts with elongated hypocotyl5 (HY5) and constitutive photomorphogenic1 (COP1) and promotes COP1-mediated degradation of HY5 during arabidopsis seedling development[J]. Plant Physiology, 2015, 169(4): 2922–2934. |
 2018, Vol. 26
2018, Vol. 26



