2. 中国科学院大学 北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049, China
环渤海地区是我国重要的经济发展区, 但由于该地区分布有大量的盐碱荒地, 土壤贫瘠、植被稀疏、生态环境恶劣[1], 迫切需要加快当地的植被建设, 以满足社会经济快速发展需求[2]。在盐碱地植被建设中, 客土绿化由于成本高、可持续性差等局限性[3], 难于大面积应用到盐碱地的生态治理。相比之下, 利用耐盐植物重建植被, 完成原土绿化日益受到重视。
碱蓬(Suaeda glauca)和盐地碱蓬(Suaeda salsa)属于藜科(Chenopodiaceae)碱蓬属的一年生草本, 是我国本土盐生植物。研究表明种植碱蓬、盐地碱蓬具有改善土壤性状、降低土壤含盐量、修复土壤重金属污染、净化富营养水体等多种生态效益[3-8]。碱蓬、盐地碱蓬作为重要的物种资源, 在盐碱地的生态治理与修复领域具有重要的开发潜力。碱蓬、盐地碱蓬在自然界的分布具有明显的地带性:碱蓬多分布于东北、西北等内陆盐碱地区[9], 盐地碱蓬在辽河三角洲、黄河三角洲等滨海地区的分布明显多于碱蓬; 在小地形区域, 碱蓬多分布于土坡、沙丘的高处, 盐地碱蓬多分布于低洼地带[10]。
在盐碱地中, 种子萌发是植物生长与种群建成的重要阶段, 同时也是对逆境的敏感时期[11-13]。植物萌发阶段受到的胁迫主要包括干旱胁迫、盐胁迫和碱胁迫。盐地碱蓬作为典型的盐生植物, 前人关于盐地碱蓬的报道多局限于盐胁迫[14-17], 已有研究表明盐地碱蓬对NaCl具有高度的适应性, 离子区隔化与叶片肉质化是盐地碱蓬重要的耐盐机制[18-19]。碱蓬作为盐地碱蓬的近缘物种, 其形态与盐地碱蓬多有相似之处, 但目前鲜有对碱蓬逆境生理研究的报道。因此, 本文利用PEG 6000、NaCl、Na2CO3分别模拟旱、盐、碱胁迫, 对碱蓬、盐地碱蓬种子萌发及胚的生长特性进行比较研究, 旨在探讨二者地带性分布的可能机制, 为碱蓬、盐地碱蓬在盐碱地上的种群建植提供理论依据。
1 材料与方法 1.1 试验材料碱蓬和盐地碱蓬种子于2016年11月采自河北省海兴县盐碱地(117°32′~117°58′E, 38°19′~38°29′N), 经干燥和清理后, 保存于纸袋, 放置于4 ℃冰箱保存。
1.2 试验设计挑选饱满、大小均匀的碱蓬、盐地碱蓬黑色种子, 在5%次氯酸钠溶液中浸泡5 min, 随后用蒸馏水冲洗除去残留, 置于铺2层滤纸的直径90 mm的培养皿中, 每个培养皿放50粒种子, 加入处理液10 mL, 各处理3个重复。试验设计以蒸馏水处理为对照, 以PEG 6000、NaCl、Na2CO3溶液为处理组, 分别配制-0.46 MPa、-0.92 MPa、-1.38 MPa、-1.84 MPa 4个溶液渗透势梯度。由WP4C露点水势仪(美国Decagon公司)测定溶液的渗透势、pH仪(Sartorius, PB-10)测定溶液的pH(表 1)。本文所有试验均在人工智能气候箱中完成, 光照14 h·d-1, 光强≥56.6 μmol·s-1, 温度25 ℃/20 ℃(昼/夜), 相对湿度75%~80%。每24 h统计种子萌发数, 以可见胚根为萌发标准。第7 d测量已萌发种子的胚轴与胚根长度, 拍照, 并将未萌发种子清洗后移至蒸馏水处理复水, 7 d后计算种子最终萌发数。
| 表1 不同胁迫处理液的浓度、渗透势和pH Table 1 Concentration, osmotic potential and pH of the treatment solutions |
| $ 萌发率=(试验种子萌发个数/待测种子总数)×100\% $ | (1) |
| $ 最终萌发率=[(试验种子萌发个数+复水后新萌发的 \\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ 种子个数)/待测种子总数]×100\% $ | (2) |
| $ 萌发指数=∑(第t天种子萌发个数/相应的萌发天数t) $ | (3) |
| $ 平均萌发时间=∑(第t天种子萌发个数×相应的萌发 \\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ 天数t)/试验种子萌发个数 $ | (4) |
采用SPSS 16.0软件对实验数据进行邓肯多重比较, P < 0.05为差异显著, 用Origin 9.1作图。
2 结果与分析 2.1 PEG、NaCl、Na2CO3胁迫对碱蓬、盐地碱蓬种子萌发率、最终萌发率的影响由图 1可知, 碱蓬和盐地碱蓬种子在蒸馏水处理中的萌发率分别为96.7%和84.7%。低渗处理(-0.46 MPa)下, PEG、NaCl和Na2CO3对碱蓬和盐地碱蓬的种子萌发无显著影响。-0.92 MPa NaCl处理的碱蓬和盐地碱蓬种子的萌发率与对照无显著差异; 等渗的PEG、Na2CO3处理未显著影响盐地碱蓬种子的萌发, 但显著抑制了碱蓬种子的萌发, 且Na2CO3的抑制作用大于PEG。高渗处理(-1.38 MPa、-1.84 MPa)明显抑制碱蓬、盐地碱蓬种子的萌发率, 且溶液渗透势越低, 种子萌发受抑制程度越大。-1.38 MPa NaCl处理下, 碱蓬和盐地碱蓬种子萌发率分别比对照降低16.7%和14.7%。高渗PEG、Na2CO3处理对碱蓬种子萌发率的抑制作用明显大于盐地碱蓬, -1.38 MPa PEG、Na2CO3处理中碱蓬种子的萌发率比对照降低84.7%和86.7%, 同处理条件下, 盐地碱蓬种子萌发率比对照分别降低28.0%和30.7%。
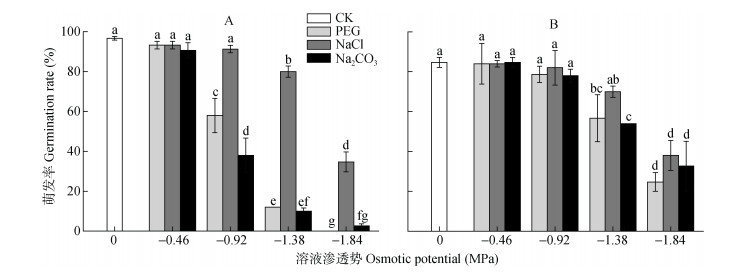
|
图 1 不同渗透势PEG、NaCl和Na2CO3处理对碱蓬(A)和盐地碱蓬(B)萌发率的影响 Figure 1 Effects of PEG, NaCl and Na2CO3 with different ostimotic potentials on the germination rates of Suaeda glauca (A) and Suaeda salsa (B) 不同小写字母表示不用处理间0.05水平差异显著。 Different lowercase letters mean significant differences among treatments at P < 0.05. |
复水7 d后, 所有处理组碱蓬和盐地碱蓬种子的最终萌发率与对照均无显著差异(图 2), 但随着Na2CO3胁迫的加重, 盐地碱蓬种子的最终萌发率呈现下降的趋势, -1.38 MPa和-1.84 MPa Na2CO3处理下, 盐地碱蓬种子最终萌发率比对照分别降低7.1%和15.8%。
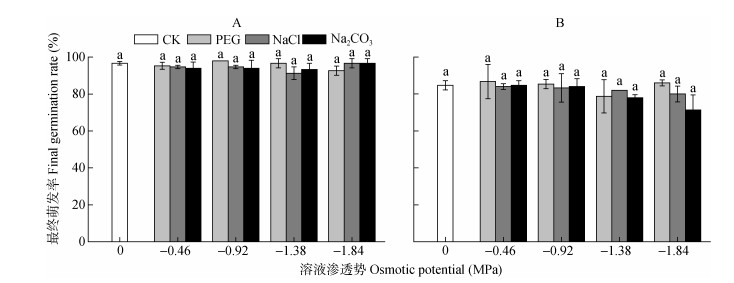
|
图 2 不同渗透势PEG、NaCl和Na2CO3处理对碱蓬(A)和盐地碱蓬(B)最终萌发率的影响 Figure 2 Effects of PEG, NaCl and Na2CO3 with different ostimotic potentials on the final germination rates of Suaeda glauca (A) and Suaeda salsa (B) 不同小写字母表示不用处理间0.05水平差异显著。 Different lowercase letters mean significant differences among treatments at P < 0.05. |
萌发指数是种子重要的活力指标, 种子活力由遗传因素决定, 但环境因素决定种子活力的现实性, 因此萌发指数可以很好地反映逆境对种子萌发的影响程度[20-21]。碱蓬、盐地碱蓬种子萌发指数随溶液渗透势的降低而降低, 不同处理组种子的萌发指数与溶液渗透势均呈线性相关关系(图 3)。参考回归方程的斜率发现:溶液渗透势每下降1 MPa, PEG、NaCl、Na2CO3处理组碱蓬种子的萌发指数分别下降11.20、7.24、10.76, 碱蓬种子的萌发指数在NaCl处理中随溶液渗透势下降的程度明显小于PEG和Na2CO3处理; 对于盐地碱蓬, 溶液渗透势每下降1 MPa, PEG、NaCl、Na2CO3处理组种子的萌发指数分别下降16.38、17.12、15.82, 各处理间种子萌发指数变化趋势相似。碱蓬、盐地碱蓬萌发指数对PEG、NaCl、Na2CO3胁迫的响应与萌发率部分的结论高度一致。
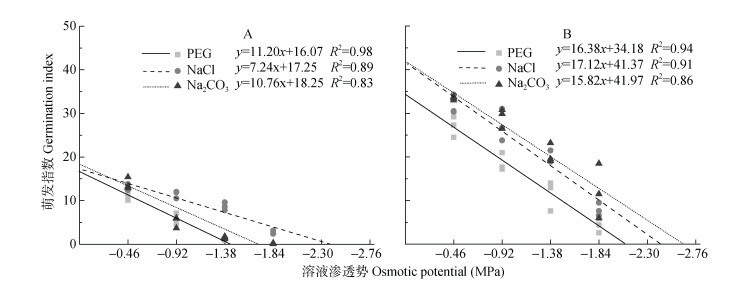
|
图 3 不同渗透势PEG、NaCl和Na2CO3处理对碱蓬(A)和盐地碱蓬(B)萌发指数的影响 Figure 3 Effects of PEG, NaCl and Na2CO3 with different ostimotic potentials on the germination indexes of Suaeda glauca (A) and Suaeda salsa (B) |
由图 4可知, 对照组碱蓬和盐地碱蓬种子的平均萌发时间分别为3.5 d和1.7 d。随着PEG胁迫的加重, 碱蓬、盐地碱蓬种子平均萌发时间逐渐升高。当溶液渗透势为-0.92 MPa、-1.38 MPa时, NaCl、Na2CO3处理组碱蓬、盐地碱蓬种子的平均萌发时间明显小于等渗PEG处理, 并且与对照无显著差异。
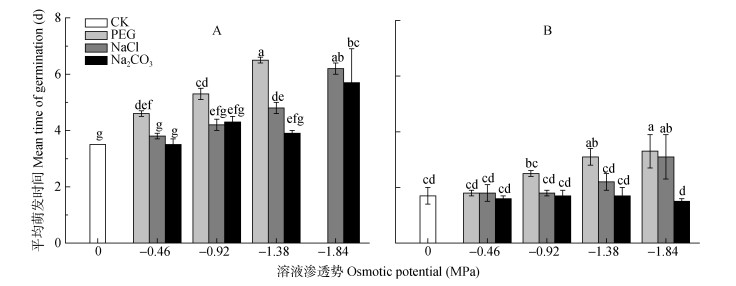
|
图 4 不同渗透势PEG、NaCl和Na2CO3处理对碱蓬(A)和盐地碱蓬(B)平均萌发时间的影响 Figure 4 Effects of PEG, NaCl and Na2CO3 with different ostimotic potentials on the mean germination times of Suaeda glauca (A) and Suaeda salsa (B) 不同小写字母表示不用处理间0.05水平差异显著。 Different lowercase letters mean significant differences among treatments at P < 0.05. |
在PEG和Na2CO3处理中, 碱蓬、盐地碱蓬胚轴、胚根长度随溶液渗透势降低而下降, 低渗NaCl处理(-0.46 MPa)处理对碱蓬、盐地碱蓬胚的生长具有促进作用, 高渗NaCl处理(-1.38 MPa、-1.84 MPa)对碱蓬和盐地碱蓬胚轴、胚根的生长具有抑制作用, 但NaCl处理对碱蓬和盐地碱蓬胚生长的抑制作用明显小于等渗PEG和Na2CO3处理(图 5, 图 6)。碱蓬、盐地碱蓬胚的生长对NaCl和Na2CO3胁迫的响应存在差异: -0.92 MPa NaCl处理对盐地碱蓬胚根生长具有明显的促进作用, 而碱蓬胚根的生长受到抑制; -0.46 MPa Na2CO3处理下, 碱蓬的胚轴和胚根长度分别比对照降低13.0%和12.9%, 而盐地碱蓬胚轴和胚根长分别降低33.9%和64.0%, -0.46 MPa Na2CO3处理对盐地碱蓬胚的生长抑制作用大于碱蓬(图 5, 图 6)。
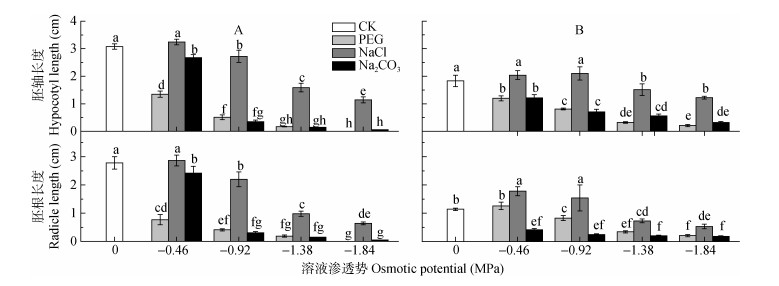
|
图 5 不同渗透势PEG、NaCl和Na2CO3处理对碱蓬(A)和盐地碱蓬(B)胚轴、胚根长度的影响 Figure 5 Effects of PEG, NaCl and Na2CO3 with different ostimotic potentials on the hypocotyl and radicle lengths of Suaeda glauca (A) and Suaeda salsa (B) 不同小写字母表示不用处理间0.05水平差异显著。 Different lowercase letters mean significant differences among treatments at P < 0.05. |

|
图 6 不同渗透势PEG、NaCl和Na2CO3处理对碱蓬和盐地碱蓬幼苗形成的影响 Figure 6 Effects of PEG, NaCl and Na2CO3 with different ostimotic potentials on the seedlings establishment of Suaeda glauca and Suaeda salsa |
植物的抗逆性不仅取决于植物种类, 在不同的生长阶段, 植物的抗逆性也存在较大差异。植物在种子萌发期与幼苗形成期对逆境敏感, 成株抗性普遍较高[22], 因此种子萌发与幼苗形成期是研究植物抗逆性的最佳时期。与棉花(Gossypium spp.)、茄子(Solanum melongena)、芝麻(Sesamum indicum)的研究一致[23-25], 碱蓬、盐地碱蓬在种子萌发时期抗性较高, 在幼苗形成期的抗逆性明显低于种子萌发期。本研究表明, 低渗PEG、Na2CO3处理(-0.46 MPa)对种子萌发率影响不显著, 但对碱蓬、盐地碱蓬胚的生长产生显著的抑制作用; 高渗PEG、NaCl、Na2CO3胁迫(-1.38 MPa、-1.84 MPa)对碱蓬、盐地碱蓬的萌发与生长均产生不同程度的抑制作用。
种子萌发率与萌发指数结果表明:在等渗条件下, PEG与Na2CO3胁迫对碱蓬种子萌发的抑制作用无显著差异, NaCl胁迫对碱蓬种子萌发的抑制程度明显小于等渗PEG、Na2CO3胁迫; 盐地碱蓬种子的萌发主要受渗透胁迫的影响, 对PEG、NaCl、Na2CO3胁迫的响应无显著差异。有研究表明, 在等渗条件下细茎针茅(Stipa tenacissima)、长角豆(Ceratonia siliqua)种子在NaCl溶液中的萌发率高于PEG[26-27]。本研究中碱蓬、盐地碱蓬也出现类似现象, 这可能是由于NaCl起到了渗透调节作用。NaCl作为无机离子, 在机体的富集有助于减缓渗透胁迫的负面影响[28]。Cavallaro等[29]研究表明长角豆种子在NaCl溶液中的吸胀速率明显高于在等渗PEG溶液中。NaCl胁迫下碱蓬、盐地碱蓬种子的吸胀与萌动过程还有待深入研究。对牛至(Origanum compactum)、羊草(Leymus chinensis)的研究表明, 高pH是抑制种子萌发的重要因素[30-31]。本研究中尽管Na2CO3胁迫对碱蓬、盐地碱蓬种子萌发产生了明显的抑制作用, 但在等渗条件下, Na2CO3胁迫与PEG胁迫的抑制作用无显著差异。由此可知, 对于碱蓬、盐地碱蓬, Na2CO3胁迫对种子萌发的抑制作用主要是由于渗透胁迫, 高pH胁迫没有产生显著影响。
植物种子在高盐逆境下存活, 雨季后集中萌发并建立种群是盐生植物适应盐生境的重要策略[32-33]。大部分盐生植物种子具有较强的恢复萌发能力, 也有物种如驼蹄瓣(Zygophyllum simplex)、异子蓬(Borszczowia aralocaspica)、地肤(Kochia scoparia)种子的萌发活性被盐胁迫永久抑制[34-36]。复水试验表明PEG、NaCl胁迫下碱蓬、盐地碱蓬种子均具有良好的恢复萌发能力, 虽然Na2CO3处理组种子的复水萌发率与对照差异不显著, 但Na2CO3胁迫有降低盐地碱蓬种子最终萌发率的趋势, Na2CO3胁迫对盐地碱蓬种子产生了毒害作用。
对碱蓬、盐地碱蓬早期生长特性的研究表明, 碱蓬、盐地碱蓬均表现喜盐的特征, 但盐地碱蓬对NaCl的抗性高于碱蓬。轻度NaCl处理对盐地碱蓬胚根伸长的促进作用明显大于碱蓬, 高NaCl处理对盐地碱蓬根长的抑制作用小于碱蓬, 这与前人研究结果一致[37]。与盐胁迫相比, 碱胁迫还会对植物产生高pH伤害, 金属离子与磷的沉淀会阻碍植物对矿质营养的吸收, 从而扰乱机体的离子平衡与pH稳态[38-39], 对植物产生更严重的损伤。与苍耳(Xanthium sibiricum)、碱地肤(Kochia sieversiana)、灰绿藜(Chenopodium glaucum)[40-42]等盐生植物一样, Na2CO3胁迫对碱蓬、盐地碱蓬生长的抑制作用明显大于NaCl胁迫, 重度碱胁迫(-0.92 MPa、-1.38 MPa、-1.84 MPa)下, 碱蓬、盐地碱蓬根尖出现变黑死亡的现象。比较碱蓬与盐地碱蓬发现, 二者在幼苗形成期对轻度Na2CO3胁迫(-0.46 MPa)的抗性存在差异, 轻度Na2CO3胁迫(-0.46 MPa)对碱蓬胚生长的抑制作用明显小于盐地碱蓬。碱蓬与盐地碱蓬在幼苗形成期抗盐、抗碱性的差异可能是导致二者地带性分布的重要原因。我国东北盐渍区属于苏打碱土, 西北黄河中上游盐渍土主要盐分为碳酸盐, 土壤都明显偏碱性[43-44], 碱蓬对轻度Na2CO3胁迫的抗性优于盐地碱蓬, 因此碱蓬更偏向分布于内陆轻度碱土地区; 而辽河、黄河等滨海盐碱地是土壤盐分以NaCl为主的盐土, 盐地碱蓬对NaCl的高度适应性可能是盐地碱蓬种群从内陆向沿海发展以及低洼地带分布的重要原因。
本研究通过对碱蓬、盐地碱蓬在种子萌发及幼苗形成期逆境响应的综合分析, 发现碱蓬和盐地碱蓬对旱、盐、碱胁迫的响应趋势基本一致, 碱蓬和盐地碱蓬均具有很强的抗盐性, 并且抗盐能力明显高于抗旱、抗碱能力。比较碱蓬和盐地碱蓬, 在种子萌发期, 碱蓬种子的抗旱、抗碱能力低于盐地碱蓬; 在幼苗形成期, 碱蓬的抗盐性小于盐地碱蓬, 但对轻度碱胁迫的抗性高于盐地碱蓬。
| [1] |
毛建华, 刘太祥, 刘洪庆, 等. 滨海盐土绿化的排盐改土技术规程编制说明[J]. 天津农业科学, 2011, 17(3): 29–31.
MAO J H, LIU T X, LIU H Q, et al. Direction for drawing up technology standard for the drainage, salt-leaching and soil-reclamation in greening coastal saline land[J]. Tianjin Agricultural Sciences, 2011, 17(3): 29–31. |
| [2] |
胡培兴. 京津风沙源成因分析与防治对策研究[D]. 南京: 南京林业大学, 2007: 35-46
HU P X. The causes, evaluation and strategies of desertification in Beijing and Tianjin regions in P. R. China[D]. Nanjing: Nanjing Forestry University, 2007: 35-46 http://cdmd.cnki.com.cn/article/cdmd-10298-2007204497.htm |
| [3] |
林学政, 沈继红, 刘克斋, 等. 种植盐地碱蓬修复滨海盐渍土效果的研究[J]. 海洋科学进展, 2005, 23(1): 65–69.
LIN X Z, SHEN J H, LIU K Z, et al. Study on remediation effects of Suaeda salsa L. planting on coastal saline soil[J]. Advances in Marine Science, 2005, 23(1): 65–69. |
| [4] |
李超峰, 葛宝明, 姜森颢, 等. 碱蓬对盐碱及污染土壤生物修复的研究进展[J]. 土壤通报, 2014, 45(4): 1014–1019.
LI C F, GE B M, JIANG S H, et al. Review on remedial effect of Suaeda salsa on saline and polluted soils[J]. Chinese Journal of Soil Science, 2014, 45(4): 1014–1019. |
| [5] |
邹桂梅, 苏德荣, 黄明勇, 等. 人工种植盐地碱蓬改良吹填土的试验研究[J]. 草业科学, 2010, 27(4): 51–56.
ZOU G M, SU D R, HUANG M Y, et al. Effect of planting Suaeda salsa on improvement of dredger filled soil[J]. Pratacultural Science, 2010, 27(4): 51–56. |
| [6] |
张亚, 常雅军, 刘晓静, 等. 碱蓬对不同盐度富营养化模拟海水的净化效应及其生长特性[J]. 植物资源与环境学报, 2016, 25(4): 34–41.
ZHANG Y, CHANG Y J, LIU X J, et al. Purification effect of Suaeda glauca on eutrophic simulated seawater with different salt concentrations and its growth character[J]. Journal of Plant Resources and Environment, 2016, 25(4): 34–41. |
| [7] |
李从娟, 孙永强, 范敬龙, 等. 盐地碱蓬在高盐碱土环境中的生态学意义[J]. 干旱区研究, 2015, 32(6): 1160–1166.
LI C J, SUN Y Q, FAN J L, et al. Ecological significance of planting Suaeda salsa in saline/alkali soils in the Lop Nur Potash Mine[J]. Arid Zone Research, 2015, 32(6): 1160–1166. |
| [8] | ZHAO K F. Desalinization of saline soils by Suaeda salsa[J]. Plant and Soil, 1991, 135(2): 303–305. DOI:10.1007/BF00010921 |
| [9] |
杜晓光, 郑慧莹, 刘存德. 松嫩平原主要盐碱植物群落生物生态学机制的初步探讨[J]. 植物生态学报, 1994, 18(1): 41–49.
DU X G, ZHENG H Y, LIU C D. A preliminary study on the main plant communities in the saline soils of Song-Nen Plain[J]. Acta Phytoecologica Sinica, 1994, 18(1): 41–49. |
| [10] | CUI B S, HE Q, ZHAO X S. Ecological thresholds of Suaeda salsa to the environmental gradients of water table depth and soil salinity[J]. Acta Ecologica Sinica, 2008, 28(4): 1408–1418. DOI:10.1016/S1872-2032(08)60050-5 |
| [11] | SHAYGAN M, BAUMGARTL T, ARNOLD S. Germination of Atriplex halimus seeds under salinity and water stress[J]. Ecological Engineering, 2017, 102: 636–640. DOI:10.1016/j.ecoleng.2017.02.050 |
| [12] | LIU H L, ZHANG D Y, YANG X J, et al. Seed dispersal and germination traits of 70 plant species inhabiting the Gurbantunggut desert in Northwest China[J]. The Scientific World Journal, 2014, 2014: 346405. |
| [13] | MUNNS R, TESTER M. Mechanisms of salinity tolerance[J]. Annual Review of Plant Biology, 2008, 59: 651–681. DOI:10.1146/annurev.arplant.59.032607.092911 |
| [14] | LI W Q, LIU X J, KHAN M A, et al. The effect of plant growth regulators, nitric oxide, nitrate, nitrite and light on the germination of dimorphic seeds of Suaeda salsa under saline conditions[J]. Journal of Plant Research, 2005, 118(3): 207–214. DOI:10.1007/s10265-005-0212-8 |
| [15] | WANG F X, XU Y G, WANG S, et al. Salinity affects production and salt tolerance of dimorphic seeds of Suaeda salsa[J]. Plant Physiology and Biochemistry, 2015, 95: 41–48. DOI:10.1016/j.plaphy.2015.07.005 |
| [16] | SONG J, WANG B S. Using euhalophytes to understand salt tolerance and to develop saline agriculture:Suaeda salsa as a promising model[J]. Annals of Botany, 2015, 115(3): 541–553. DOI:10.1093/aob/mcu194 |
| [17] |
段德玉, 刘小京, 冯凤莲, 等. 不同盐分胁迫对盐地碱蓬种子萌发的效应[J]. 中国农学通报, 2003, 19(6): 168–172.
DUAN D Y, LIU X J, FENG F L, et al. Effect of salinities on seed germination of halophyte Suaeda Salsa[J]. Chinese Agricultural Science Bulletin, 2003, 19(6): 168–172. |
| [18] | WANG B S, LÜTTGE U, RATAJCZAK R. Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa[J]. Journal of Experimental Botany, 2001, 52(365): 2355–2365. DOI:10.1093/jexbot/52.365.2355 |
| [19] | YANG M F, SONG J, WANG B S. Organ-specific responses of vacuolar H+-ATPase in the shoots and roots of C3 halophyte Suaeda salsa to NaCl[J]. Journal of Integrative Plant Biology, 2010, 52(3): 308–314. DOI:10.1111/jipb.2010.52.issue-3 |
| [20] |
闫兴富, 周立彪, 思彬彬, 等. 不同温度下PEG-6000模拟干旱对柠条锦鸡儿种子萌发的胁迫效应[J]. 生态学报, 2016, 36(7): 1989–1996.
YAN X F, ZHOU L B, SI B B, et al. Stress effects of simulated drought by polyethylene glycol on the germination of Caragana korshinskii Kom. seeds under different temperature conditions[J]. Acta Ecologica Sinica, 2016, 36(7): 1989–1996. |
| [21] | ZHANG H X, IRVING L J, MCGILL C, et al. The effects of salinity and osmotic stress on barley germination rate:Sodium as an osmotic regulator[J]. Annals of Botany, 2010, 106(6): 1027–1035. DOI:10.1093/aob/mcq204 |
| [22] | DODD G L, DONOVAN L A. Water potential and ionic effects on germination and seedling growth of two cold desert shrubs[J]. American Journal of Botany, 1999, 86(8): 1146–1153. DOI:10.2307/2656978 |
| [23] |
谢德意, 王惠萍, 王付欣, 等. 盐胁迫对棉花种子萌发及幼苗生长的影响[J]. 中国棉花, 2000, 27(9): 12–13.
XIE D Y, WANG H P, WANG F X, et al. Effects of cotton seeds germination and seedling growth under salt stress[J]. China Cotton, 2000, 27(9): 12–13. |
| [24] | DEMIR I, MAVI K, OZCOBAN M, et al. Effect of salt stress on germination and seedling growth in serially harvested aubergine (Solanum melongena L.) seeds during development[J]. Israel Journal of Plant Sciences, 2003, 51(2): 125–131. DOI:10.1560/EAD8-YE43-E5BE-WHHV |
| [25] | HARFI M E, HANINE H, RIZKI H, et al. Effect of drought and salt stresses on germination and early seedling growth of different color-seeds of sesame (Sesamum indicum)[J]. International Journal of Agriculture and Biology, 2016, 18(6): 1088–1094. DOI:10.17957/IJAB |
| [26] | KRICHEN K, VILAGROSA A, CHAIEB M. Environmental factors that limit Stipa tenacissima L. germination and establishment in Mediterranean arid ecosystems in a climate variability context[J]. Acta Physiologiae Plantarum, 2017, 39: 175. DOI:10.1007/s11738-017-2475-9 |
| [27] | CAVALLARO V, BARBERA A C, MAUCIERI C, et al. Evaluation of variability to drought and saline stress through the germination of different ecotypes of carob (Ceratonia siliqua L.) using a hydrotime model[J]. Ecological Engineering, 2016, 95: 557–566. DOI:10.1016/j.ecoleng.2016.06.040 |
| [28] | LI R, SHI F, FUKUDA K. Interactive effects of salt and alkali stresses on seed germination, germination recovery, and seedling growth of a halophyte Spartina alterniflora (Poaceae)[J]. South African Journal of Botany, 2010, 76(2): 380–387. DOI:10.1016/j.sajb.2010.01.004 |
| [29] | CAVALLARO V, BARBERA A C, MAUCIERI C, et al. Evaluation of variability to drought and saline stress through the germination of different ecotypes of carob (Ceratonia siliqua L.) using a hydrotime model[J]. Ecological Engineering, 2016, 95: 557–566. DOI:10.1016/j.ecoleng.2016.06.040 |
| [30] | LAGHMOUCHI Y, BELMEHDI O, BOUYAHYA A, et al. Effect of temperature, salt stress and pH on seed germination of medicinal plant Origanum compactum[J]. Biocatalysis and Agricultural Biotechnology, 2017, 10: 156–160. DOI:10.1016/j.bcab.2017.03.002 |
| [31] | MA H Y, YANG H Y, LÜ X T, et al. Does high pH give a reliable assessment of the effect of alkaline soil on seed germination? A case study with Leymus chinensis (Poaceae)[J]. Plant and Soil, 2015, 394(1/2): 35–43. |
| [32] | SONG J, FENG G, ZHANG F S. Salinity and temperature effects on germination for three salt-resistant euhalophytes, Halostachys caspica, Kalidium foliatum and Halocnemum strobilaceum[J]. Plant and Soil, 2006, 279(1/2): 201–207. |
| [33] | SONG J, FENG G, TIAN C Y, et al. Strategies for adaptation of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum to a saline environment during seed-germination stage[J]. Annals of Botany, 2005, 96(3): 399–405. DOI:10.1093/aob/mci196 |
| [34] | KHAN M A, GUL B, WEBER D J. Germination responses of Salicornia rubra to temperature and salinity[J]. Journal of Arid Environments, 2000, 45(3): 207–214. DOI:10.1006/jare.2000.0640 |
| [35] | KHAN M A, UNGAR I A. Effects of thermoperiod on recovery of seed germination of halophytes from saline conditions[J]. American Journal of Botany, 1997, 84(2): 279–283. DOI:10.2307/2446089 |
| [36] | ZHANG H X, ZHANG G M, LÜ X T, et al. Salt tolerance during seed germination and early seedling stages of 12 halophytes[J]. Plant and Soil, 2015, 388(1/2): 229–241. |
| [37] |
弋良朋, 王祖伟. 盐胁迫下3种滨海盐生植物的根系生长和分布[J]. 生态学报, 2011, 31(5): 1195–1202.
YI L P, WANG Z W. Root system characters in growth and distribution among three littoral halophytes[J]. Acta Ecologica Sinica, 2011, 31(5): 1195–1202. |
| [38] | SHI D C, ZHAO K F. Effects of NaCl and Na2CO3 on growth of Puccinellia tenuiflora and on present state of mineral elements in nutrient solution[J]. Acta Prataculturae Sinica, 1997, 6(2): 51–61. |
| [39] | YANG C W, CHONG J N, LI C Y, et al. Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions[J]. Plant and Soil, 2007, 294(1/2): 263–276. |
| [40] |
刘强, 王占武, 周晓梅. 苍耳对盐碱胁迫的生理响应[J]. 东北林业大学学报, 2017, 45(4): 23–27.
LIU Q, WANG Z W, ZHOU X M, et al. Physiological responses of Xanthium sibiricum to salt and alkali stresses[J]. Journal of Northeast Forestry University, 2017, 45(4): 23–27. |
| [41] |
贾娜尔·阿汗, 杨春武, 石德成, 等. 盐生植物碱地肤对盐碱胁迫的生理响应特点[J]. 西北植物学报, 2007, 27(1): 79–84.
JIANAER·Ahan, YANG C W, SHI D C, et al. Physiological response of an alkali resistant halophyte Kochia sieversiana to salt and alkali stresses[J]. Acta Botanica Boreali-Occidentalia Sinica, 2007, 27(1): 79–84. |
| [42] |
古丽内尔·亚森, 杨瑞瑞, 曾幼玲. 混合盐碱胁迫对灰绿藜(Chenopodium glaucum L.)种子萌发的影响[J]. 生态学杂志, 2014, 33(1): 76–82.
GULNAR Y, YANG R R, ZENG Y L. Effects of salt-alkali mixed stresses on seed germination of the halophyte Chenopodium glaucum L.[J]. Chinese Journal of Ecology, 2014, 33(1): 76–82. |
| [43] | KAWANABE S, ZHU T C. Degeneration and conservation of Aneurolepidium chinense grassland in Northern China[J]. Journal of Japanese Society of Grassland Science, 1991, 37: 91–99. |
| [44] |
魏博娴. 中国盐碱土的分布与成因分析[J]. 水土保持应用技术, 2012(6): 27–28.
WEI B X. The analysis of the saline-alkali soil distribution in China[J]. Technology of Soil and Water Conservation, 2012(6): 27–28. |
 2018, Vol. 26
2018, Vol. 26



